Set parameter values
| Set anterior cut position | Set posterior cut position |
| Set ΔVmem Expectation | Set ΔVmem Precision |
Select amputation experiment
Initial amputation-fragment polarization profile:
Anterior, Anterior Reference Midline Reference Posterior Reference, Posterior
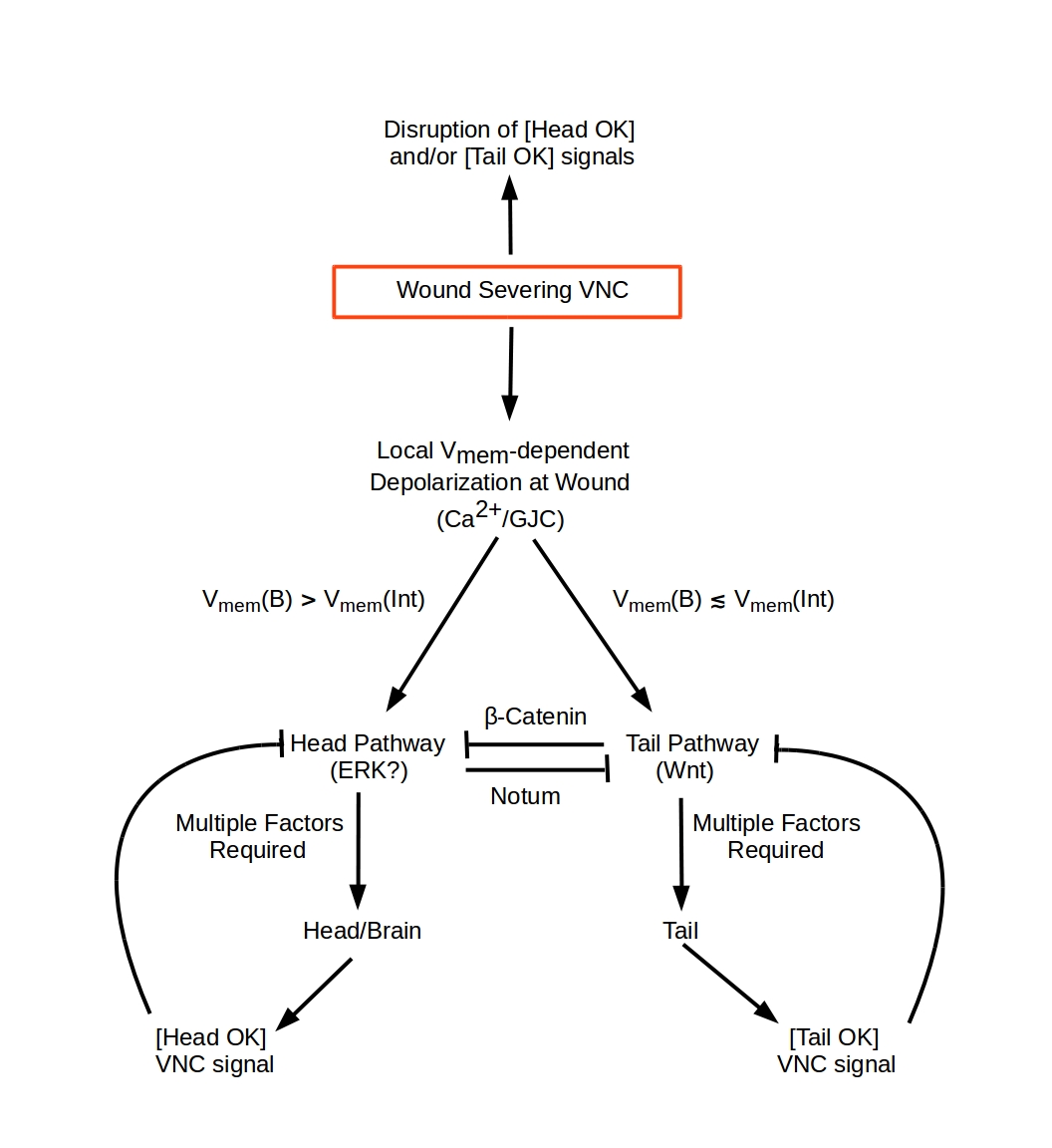 This model describes regeneration following amputations transverse to the anterior-posterior (A-P) axis in Planaria, i.e. amputations that sever both branches of the ventral nerve cord (VNC). Both single transverse wounds that remove anterior (head) or posterior (tail) structures and pairs of wounds that remove both anterior and posterior structures are considered.
The model is based on four assumptions, two concerning the encoding of A-P polarity and two concerning the behavior of cells at the wound blastema:
This model describes regeneration following amputations transverse to the anterior-posterior (A-P) axis in Planaria, i.e. amputations that sever both branches of the ventral nerve cord (VNC). Both single transverse wounds that remove anterior (head) or posterior (tail) structures and pairs of wounds that remove both anterior and posterior structures are considered.
The model is based on four assumptions, two concerning the encoding of A-P polarity and two concerning the behavior of cells at the wound blastema: